How Medical Imaging Works: The Science Behind Modern Diagnostic Radiology
Medical imaging gives clinicians a non‑invasive window into the body, helping them diagnose earlier and plan treatment more precisely. These technologies translate physical interactions—ionising X‑ray photons, magnetic resonance of atomic nuclei, and reflected ultrasound waves—into digital signals that are reconstructed as images for interpretation. Knowing the basic physics behind X‑ray and CT attenuation, MRI proton relaxation and ultrasound pulse‑echo mechanics explains why one modality is preferred over another, how contrast is created, and what trade‑offs are involved. This article walks through those principles, compares strengths and risks, highlights recent advances such as AI and photon‑counting detectors, outlines safety and comfort measures, and describes specialised clinical uses including cardiac, women’s and bone densitometry imaging. Read on for a practical framework to interpret modality choices, dose‑optimisation strategies and how new tools are changing diagnostic pathways—for both patients and referring clinicians.
How Medical Imaging Science Drives Modern Diagnostic Radiology
Imaging modalities produce contrast by different physical interactions, capture signals with specific detectors, and convert raw data into images with reconstruction algorithms. Broadly speaking, diagnostic imaging uses ionising radiation (X‑ray, CT), nuclear magnetic resonance (MRI) and acoustic reflection (ultrasound) to reveal structure and function. X‑ray and CT are fast and excellent for bone and air/soft‑tissue contrast; MRI provides superior soft‑tissue contrast through T1/T2 relaxation differences; and ultrasound offers real‑time assessment of moving structures without ionising dose. Understanding these mechanisms helps clinicians pick the right test and lets patients weigh expected risks and diagnostic benefit. Below we summarise each modality and common uses.
Choosing a modality is an optimisation between desired contrast, acceptable risk and temporal resolution. The sections that follow unpack the underlying physics and practical implications for each technique.
How Does Radiation Physics Enable X-ray and CT Imaging?
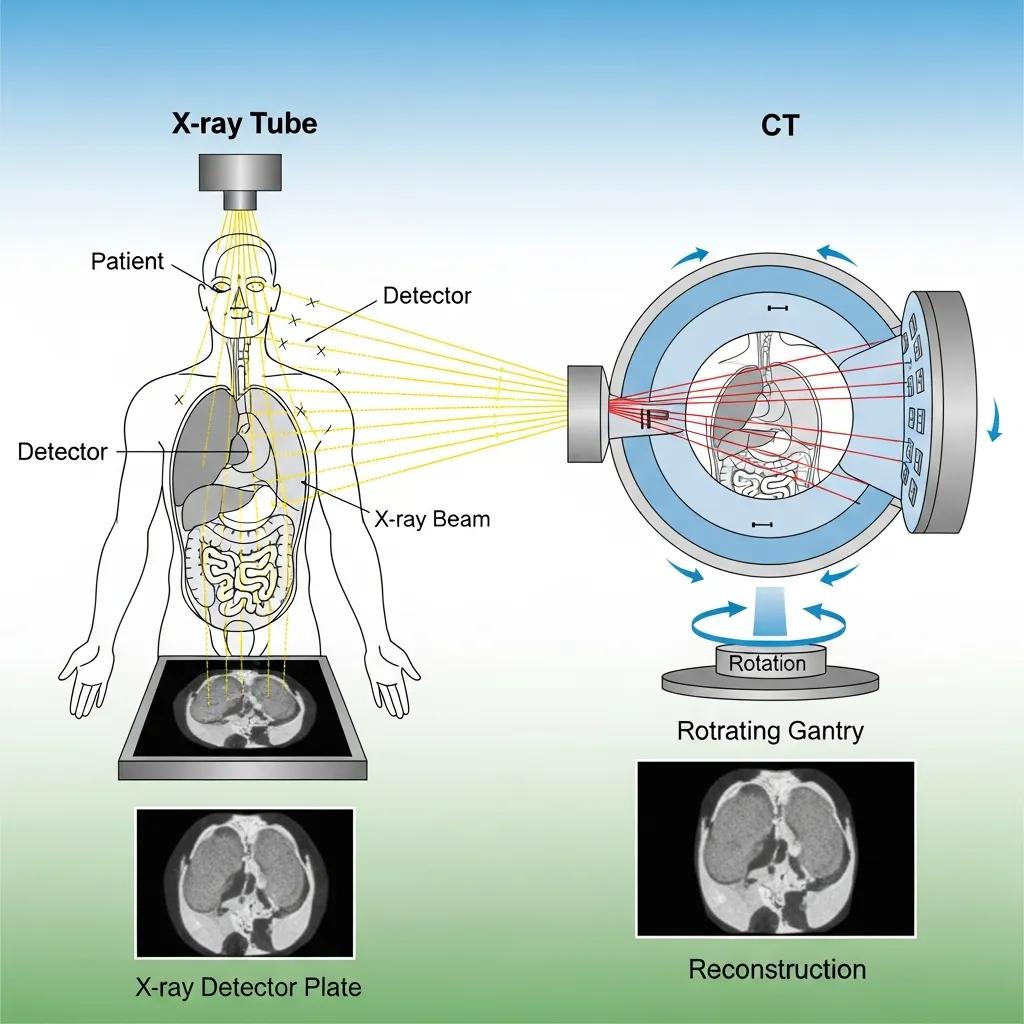
X‑ray and CT depend on how high‑energy photons are absorbed or scattered as they cross tissues: denser structures attenuate more photons, producing contrast on detectors. A plain radiograph records the integrated attenuation along each ray as a projection; CT acquires many projections around the patient and reconstructs cross‑sectional slices with algorithms such as filtered back projection or iterative reconstruction. Iodinated contrast in CT increases local attenuation to make vessels and lesions more visible. Radiation dose scales with photon flux and scan geometry; modern CT protocols employ tube current modulation, optimised voltages and advanced reconstruction to follow ALARA principles—keeping dose as low as reasonably achievable while preserving diagnostic confidence.
Detector design and reconstruction methods explain CT’s ability to deliver fast volumetric studies with fine anatomical detail, which complements the non‑ionising contrast mechanisms of MRI.
What Are the Magnetic and Radiofrequency Principles of MRI Scans?
MRI uses the magnetic properties of hydrogen nuclei. In a strong static magnetic field, many proton spins align to produce a net magnetisation. Radiofrequency (RF) pulses disturb that alignment; as protons relax back to equilibrium they emit signals that are spatially encoded by magnetic field gradients. Tissue contrast depends largely on T1 (longitudinal) and T2 (transverse) relaxation times, and sequence design weights images to emphasise these differences. Coil design and gradient performance affect resolution and scan speed, enabling specialised methods such as diffusion‑weighted imaging and functional MRI to probe tissue microstructure and physiology. MRI avoids ionising radiation but requires careful screening for implants and attention to patient comfort and acoustic noise—topics covered in the safety section.
Below is a concise overview of MR contrast mechanisms and the sequence tools used to highlight tissue differences.
Contrast Mechanisms in MR Imaging: Principles and Parameters
This review outlines key tissue parameters—proton density, T1 and T2 relaxation times—and explains how commonly used pulse sequences exploit these properties to distinguish normal and pathological tissue. It introduces related concepts such as T2* for gradient‑echo imaging, magnetisation transfer contrast, diffusion and perfusion effects, and sequence preparations like fat suppression or inversion pulses. The paper summarises how different acquisition strategies (spin‑echo, gradient‑echo, single‑echo and multi‑echo) tailor contrast for clinical applications.
Contrast mechanisms in MR imaging, 1999
How Do Ultrasound Imaging Principles Work in Diagnostic Radiology?
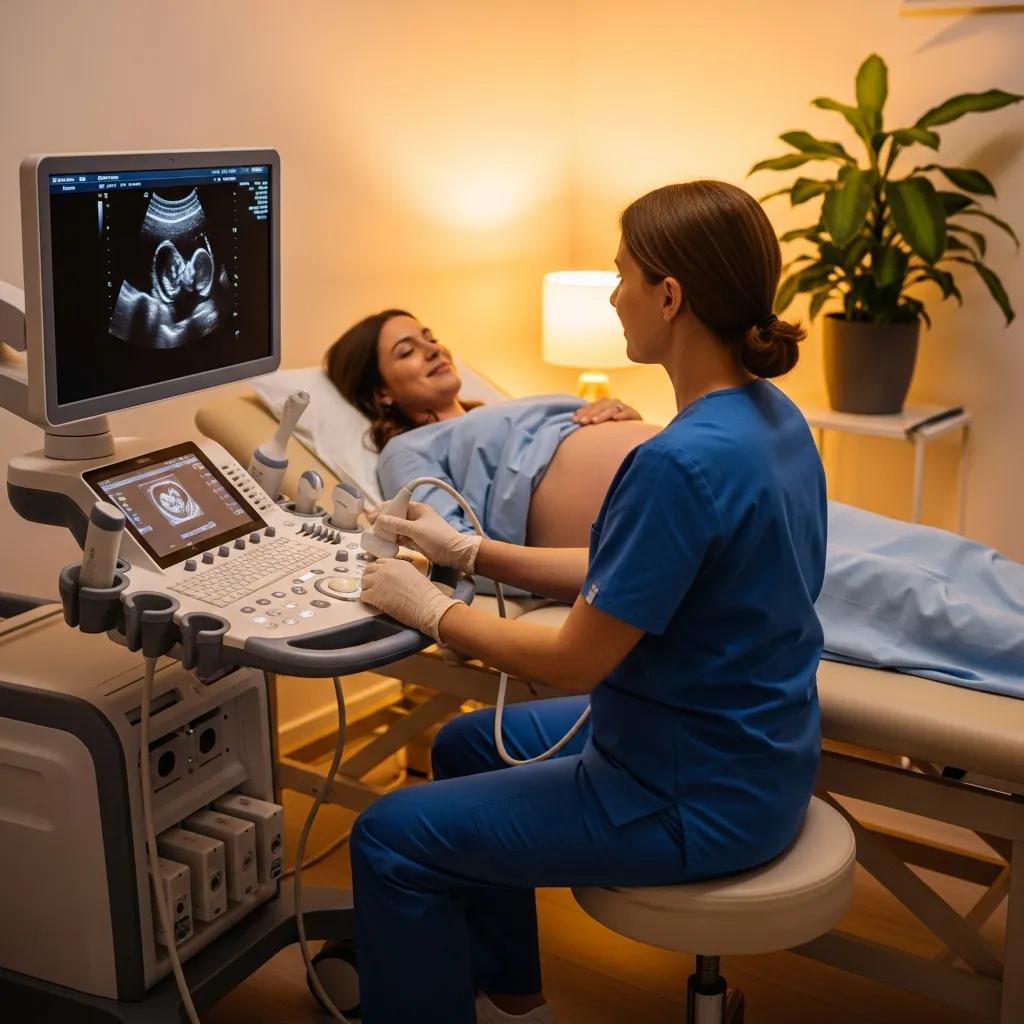
Ultrasound converts electrical energy into mechanical pressure waves using piezoelectric transducers. Waves reflect from tissue interfaces and return as echoes; the time delay maps depth while echo amplitude reflects acoustic impedance differences, producing the familiar grayscale image. Doppler processing measures frequency shifts from moving blood to assess flow speed and direction, useful in vascular studies and fetal wellbeing checks. Higher frequencies deliver better resolution but shallower penetration; lower frequencies reach deeper structures with reduced detail.
- Linear probes: High frequency, ideal for superficial vascular and musculoskeletal scans.
- Curvilinear probes: Lower frequency, wider field for abdominal and obstetric imaging.
- Phased‑array probes: Small footprint for cardiac windows and intercostal views.
Picking the right probe balances resolution and penetration and ensures diagnostic images—next we look at volumetric ultrasound methods.
What Is the Role of Sound Waves and Transducer Technology in Ultrasound?
Piezoelectric crystals in transducers generate and receive sound; the pulse‑echo cycle converts echoes into electrical signals that are beamformed and processed into an image. Beamforming and real‑time signal processing determine lateral and axial resolution, while harmonic imaging and coded excitation extend penetration and improve contrast. Multi‑element transducer arrays enable electronic steering and focusing, supporting Doppler and elastography modes to assess flow and tissue stiffness. Choosing frequency and imaging mode is a clinical decision balancing resolution, penetration and diagnostic aim.
How Do 3D and 4D Ultrasound Technologies Enhance Imaging?
3D ultrasound captures volumetric datasets by mechanically or electronically sweeping the beam and reconstructing a rendered volume; 4D adds time, showing real‑time motion within that volume. Volumetric imaging improves structural visualisation—particularly in obstetrics and complex anatomy—allows offline measurements and multiplanar reformats, and aids surgical planning. Real‑time 4D rendering helps assess fetal movement or valve motion more intuitively, improving communication with patients. These datasets demand stronger processing and storage, but for selected indications the diagnostic and patient‑experience benefits are substantial.
What Are the Latest Advancements in Diagnostic Radiology Technology?
Recent innovations are improving image quality, cutting dose and speeding interpretation. Artificial intelligence supports detection, reconstruction and workflow prioritisation; photon‑counting CT offers higher spatial resolution and spectral data with dose‑saving potential; portable and point‑of‑care devices bring imaging outside the traditional suite; and hybrid/molecular imaging deepens functional assessment. Together, these advances improve sensitivity for subtle disease, shorten time to diagnosis and enable quantitative biomarkers. Below we highlight the major trends and their clinical benefits.
Key recent advancements include:
- Artificial Intelligence (AI): Automates detection and triage, speeds reporting and enhances reconstruction.
- Photon‑Counting CT: Higher spatial resolution and material discrimination with potential dose reduction.
- Portable Imaging: Bedside ultrasound and compact CT/MRI that extend access to emergency and remote settings.
These technologies interact: AI helps interpret higher‑resolution datasets from photon‑counting CT and portable devices, which we unpack in the sections that follow.
How Is Artificial Intelligence Improving Medical Imaging Accuracy?
Machine learning models help identify patterns—lung nodules, intracranial haemorrhage, fractures—more quickly and consistently, and they automate routine quantification such as volumetry and perfusion maps. AI in reconstruction can denoise and enhance images, permitting lower acquisition doses while preserving diagnostic detail. Importantly, AI is an assistive tool: radiologists remain responsible for clinical context and final interpretation. Regulatory oversight and validation studies are expanding, and evidence shows that responsibly integrated AI can raise sensitivity and streamline workflows.
What Are the Benefits of Photon‑Counting CT and Low‑Dose Imaging?
Photon‑counting detectors register individual X‑ray photons and measure their energy, enabling intrinsic spectral imaging and improved spatial resolution over conventional detectors. The result is better material discrimination, lower image noise and potential dose reductions for the same diagnostic performance. Clinical examples include clearer coronary plaque characterisation in cardiac CT and sharper lesion detection in oncology staging. Early adopters report better diagnostic confidence and workflow gains; ongoing studies are refining dose‑sparing protocols for routine use.
Locally, Life Medical Imaging Central Coast evaluates and adopts proven radiology advances—applying advanced CT and AI‑assisted workflows where clinically appropriate across our Central Coast clinics. Our accredited, independent practice integrates sub‑specialist reporting in women’s and cardiac imaging and uses dose‑optimised protocols and modern reconstruction techniques to balance image quality and safety. If you’re considering advanced scans such as cardiac CT or a dose‑optimised body CT, our team can discuss suitability and referral pathways.
The table below summarises key technologies with their main benefits and clinical impact.
How Does Medical Imaging Science Ensure Patient Safety and Comfort?
Safety and comfort are central to modern imaging. Protocols are designed to minimise risk while maximising diagnostic yield, and equipment advances shorten scan times and reduce anxiety. Radiation safety follows ALARA—As Low As Reasonably Achievable—through optimisation of acquisition settings, shielding and appropriate justification of studies. MRI safety depends on thorough implant screening and strategies for claustrophobic patients. Comfort measures include faster sequences, open‑bore scanners, ergonomic positioning and clear communication. Below we list common safety and comfort protocols used in contemporary imaging centres and provide a comparative dose overview to put risk into perspective.
- Protocol optimisation: Tailoring parameters to the clinical question to minimise dose.
- Screening and informed consent: MRI implant checks and clear discussion of contrast risks when used.
- Shielding and positioning: Protective devices and accurate centring to reduce unnecessary exposure.
These measures reduce avoidable risk; next we outline typical radiation safety practices and dose ranges.
What Are the Radiation Safety Protocols in CT and X-ray Imaging?
Radiation protection relies on justification, optimisation and dose limitation. Justification confirms each ionising study has a clear clinical indication; optimisation adapts tube current, voltage and collimation to patient size and diagnostic needs; and advanced reconstruction or spectral techniques preserve image quality at lower doses. The table below gives approximate effective dose ranges so patients can compare common exams—values are indicative, not absolute, and individual doses vary with equipment and protocol.
How Does Technology Improve Patient Experience During MRI and Ultrasound?
MRI sequences are faster and quieter than before thanks to compressed sensing and parallel imaging; noise‑reduction hardware and two‑way communication reduce patient discomfort. Open and wide‑bore designs help those with claustrophobia, and audiovisual distraction systems support relaxation during longer studies. Ultrasound comfort is improved with ergonomic probes, warmed gel and focused, efficient exams that minimise time on the couch. Clear pre‑scan instructions and patient education reduce anxiety and improve cooperation, which in turn enhances image quality.
If you have safety or comfort concerns, Life Medical Imaging Central Coast encourages you to raise them when booking or during referral—our staff will advise on preparation, screening and sedation alternatives when clinically appropriate.
How Are Specialized Imaging Modalities Applied in Clinical Practice?
Specialised imaging adapts acquisition and interpretation to specific organs and clinical questions. Cardiac imaging uses ECG gating and optimised contrast timing for coronary and functional assessment; women’s imaging combines high‑resolution ultrasound and breast MRI for screening and problem solving; DEXA quantifies bone mineral density using dual‑energy absorption; and paediatric imaging applies child‑specific protocols and sedation policies to minimise risk. Sub‑specialist radiologists and experienced radiographers ensure protocol selection and interpretation are matched to clinical need.
Applying the right protocol and expert reporting turns imaging signals into actionable clinical information—details follow for cardiac, women’s and bone imaging.
What Scientific Principles Underpin Cardiac and Women’s Imaging?
Cardiac CT uses rapid gantry rotation, ECG gating and carefully timed contrast injection to freeze cardiac motion and visualise coronary anatomy; temporal resolution and reconstruction filters affect vessel clarity and calcium scoring. Cardiac MRI uses gated sequences and specialised pulse programs to measure ventricular volumes, tissue characterisation (T1/T2 mapping) and perfusion. Women’s imaging relies on high‑resolution ultrasound for breast and obstetric assessment, while breast MRI employs contrast‑enhanced sequences and kinetic analysis for lesion characterisation. Multimodality correlation and sub‑specialist reporting improve sensitivity and guide appropriate follow‑up or intervention.
Life Medical Imaging Central Coast offers sub‑specialist expertise in cardiac and women’s imaging across our Central Coast locations, with dedicated cardiac CT and breast/obstetric ultrasound services backed by specialist reporting to ensure accurate diagnosis and follow‑up planning.
How Does Bone Densitometry Measure Bone Mineral Density Scientifically?
DEXA (dual‑energy X‑ray absorptiometry) uses two X‑ray energy levels to separate bone from soft tissue and calculate bone mineral density (BMD). Results are reported as absolute BMD and standardised scores: the T‑score compares to a young healthy reference, and the Z‑score compares to age‑matched peers. Clinical thresholds guide management—defining osteopenia and osteoporosis—and serial DEXA scans allow monitoring of treatment response. Accuracy depends on correct positioning, calibration and consistent region‑of‑interest selection for reliable follow‑up comparisons.
That scientific basis is why DEXA remains the reference standard for assessing fracture risk and guiding osteoporosis care.
What Is the Future of Medical Imaging Science and Technology?
The near future will see AI more deeply embedded into everyday workflows, portable imaging expanding access, and hybrid/molecular imaging advancing personalised diagnostics. AI will automate triage, rapid quantification and image enhancement, while radiologists will remain essential for clinical integration and oversight. Portable scanners will bring diagnostics to emergency departments and community clinics, reshaping triage and early management. Hybrid imaging and novel molecular tracers will combine anatomical and molecular signals to improve disease characterisation and therapy selection.
These trends will influence training, regulation and workforce roles; the short forecasts below outline likely directions and research priorities.
How Will AI and Portable Imaging Shape Diagnostic Radiology by 2025?
By 2025, AI is expected to speed routine measurements, prioritise urgent studies and support reconstruction that enables lower‑dose imaging—improving throughput and earlier detection of critical findings. Portable imaging—miniaturised ultrasound and compact CT units—will broaden diagnostic reach to bedside and remote settings, enabling faster decisions in emergency care. Successful integration requires updated clinician training, robust validation and clear regulation that preserve clinical oversight and patient safety. When implemented responsibly, these advances will make imaging faster, more accessible and more focused on patient outcomes.
What Are the Research Developments in Hybrid and Molecular Imaging?
Hybrid imaging (PET/CT, PET/MRI) combines tracer‑based functional data with high‑resolution anatomy to improve lesion localisation and therapy monitoring. Emerging tracers target metabolic pathways, receptors and immune activity to personalise oncology and neurology care. Research continues on novel radiotracers, more sensitive detectors and integrated quantitative biomarkers that predict therapy response. Translational work is taking promising agents from bench to bedside, enabling earlier disease detection and more precise treatment selection. These developments point to a future where imaging not only diagnoses disease but helps guide and measure biological therapy.
As imaging science bridges physics, biology and clinical care, practices that adopt validated advances can improve local diagnostic services. Life Medical Imaging Central Coast closely follows these developments and integrates clinically proven innovations to keep our services modern and patient‑centred across the Central Coast, supporting referrals and enquiries for advanced imaging needs.
- Featured clinical advances summarised:
AI triage and reconstruction — faster processing and improved image quality.Photon‑counting CT — sharper images and spectral analysis.Hybrid/molecular imaging — deeper functional insight for therapy planning. - Workflow and patient impact key points:
Faster diagnosis through automation and point‑of‑care imaging.Lower radiation via advanced detectors and reconstruction techniques.Greater precision in therapy selection through molecular tracers and quantitative biomarkers.
This article has outlined the scientific foundations, recent innovations, safety considerations and specialised applications that define modern diagnostic radiology. The explanations and tables are intended to help clinicians, patients and referrers understand modality choice, typical risks and the technological trends shaping practice today.
Frequently Asked Questions
What are the different types of medical imaging modalities available?
Medical imaging includes several core modalities, each suited to particular diagnostic questions: X‑ray for bone and basic chest assessment; CT for fast, detailed cross‑sectional anatomy; MRI for high‑contrast soft‑tissue evaluation; and ultrasound for real‑time, radiation‑free imaging in obstetrics and vascular work. Each modality has strengths and limits, which determine the best choice for a given clinical scenario.
How does patient preparation vary for different imaging procedures?
Preparation depends on the test. For X‑ray and CT you may need to remove metal and wear a gown. MRI requires implant screening and sometimes fasting if contrast will be used. Ultrasound instructions vary—some exams ask you to drink water to fill the bladder, others require fasting. Clear pre‑appointment instructions from your imaging centre ensure the best possible images and comfort.
What advancements are being made in portable imaging technologies?
Portable imaging—hand‑held ultrasound, compact CT and smaller MRI systems—is expanding access to diagnostics at the bedside and in remote clinics. These devices speed decision‑making in emergencies and support care where traditional scanners aren’t available. As portability improves, AI integration is helping maintain image quality and diagnostic accuracy in diverse settings.
What role does artificial intelligence play in medical imaging?
AI supports radiology by flagging urgent findings, automating routine measurements and improving reconstruction to allow lower doses. While AI can increase speed and consistency, radiologists remain responsible for clinical interpretation and decision‑making. Ongoing validation and regulation ensure these tools are safe and effective in practice.
How do safety protocols differ between imaging modalities?
Protocols reflect each modality’s risks. X‑ray and CT emphasise radiation justification and optimisation to keep doses low. MRI focuses on implant screening and managing claustrophobia or acoustic noise. Ultrasound—free of ionising radiation—centres on operator skill and equipment quality. All protocols aim to protect patients while delivering diagnostic value.
What are the implications of low‑dose imaging technologies?
Low‑dose technologies, such as photon‑counting CT and advanced reconstruction algorithms, reduce radiation exposure while preserving or improving image quality. This is especially important for children and patients who need repeated scans. Low‑dose protocols help balance diagnostic needs with long‑term safety.
How can patients ensure a positive experience during imaging procedures?
Follow pre‑scan instructions, tell staff about claustrophobia or implants, and ask questions about the procedure and safety measures. Communicating concerns allows staff to arrange comfort options—open MRI scanners, sedation where appropriate, or extra explanation to ease anxiety. Being informed and prepared improves comfort and image quality.
Conclusion
Knowing the principles and recent advances in medical imaging helps clinicians and patients make informed decisions about diagnostic pathways. AI, low‑dose techniques and portable imaging are improving accuracy, access and patient experience while keeping safety at the centre. If you’d like to learn how these technologies are used in our clinics, explore the services at Life Medical Imaging Central Coast or contact our team to discuss the most appropriate imaging options for your needs.